

A family consists of a homologous element with atoms having the same number of valence electrons and thus similar chemical properties. For more detailed information about the origins of element names, see List of chemical element name etymologies. Updated on NovemOne way to classify elements is by family. Like the periodic table, the list below organizes the elements by the number of protons in their atoms it can also be organized by other properties, such as atomic weight, density, and electronegativity.
#Carbon periodic table atomic number full
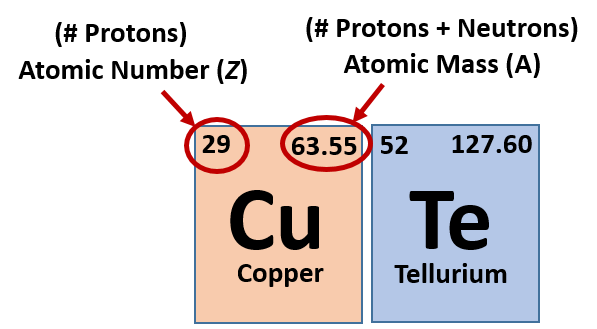
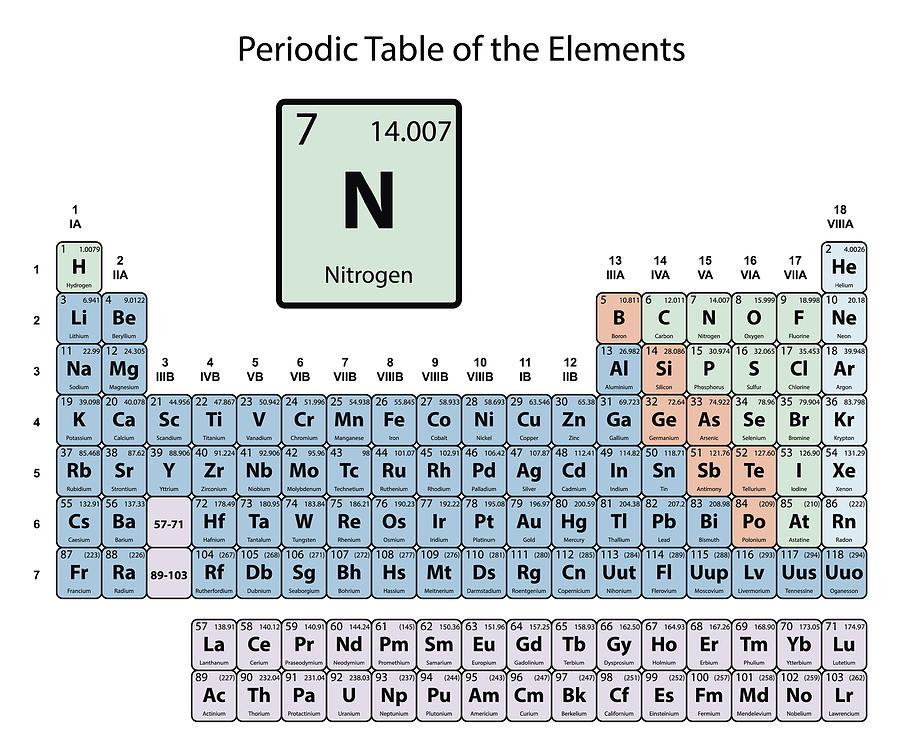
It is a tabular arrangement of the elements by their chemical properties that usually uses abbreviated chemical symbols in place of full element names, but the linear list format presented here is also useful. The definitive visualisation of all 118 elements is the periodic table of the elements, whose history along the principles of the periodic law was one of the founding developments of modern chemistry. A chemical element, often simply called an element, is a type of atom which has the same number of protons in its atomic nucleus (i.e., the same atomic number, or Z).

This is a list of the 118 chemical elements that have been identified as of 2023. Because its 2n shell is filled, it is energetically stable as a single atom and will rarely form chemical bonds with other atoms.List of the 118 identified chemical elements For instance, lithium ( Li \text Ne start text, N, e, end text ), on the other hand, has a total of ten electrons: two are in its innermost 1 s 1s 1 s 1, s orbital and eight fill the second shell-two each in the 2 s 2s 2 s 2, s and three p p p p orbitals, 1 s 2 1s^ 2 1 s 2 1, s, squared 2 s 2 2s^ 2 2 s 2 2, s, squared 2 p 6 2p^6 2 p 6 2, p, start superscript, 6, end superscript. Why is Carbon in Period 2 Let me ask you a question. This electron arrangement indicates that the outermost orbit of Carbon (C) has 4 electrons. Hence, carbon element has the electrons arrangement 2, 4. Elements in the second row of the periodic table place their electrons in the 2n shell as well as the 1n shell. Now the atomic number of Carbon (C) is 6. After the 1 s 1s 1 s 1, s orbital is filled, the second electron shell begins to fill, with electrons going first into the 2 s 2s 2 s 2, s orbital and then into the three p p p p orbitals.
#Carbon periodic table atomic number plus
The second electron shell, 2n, contains another spherical s s s s orbital plus three dumbbell-shaped p p p p orbitals, each of which can hold two electrons. Hydrogen and helium are the only two elements that have electrons exclusively in the 1 s 1s 1 s 1, s orbital in their neutral, non-charged, state. On the periodic table, hydrogen and helium are the only two elements in the first row, or period, which reflects that they only have electrons in their first shell.
This is written out as 1 s 2 1s^ 2 1 s 2 1, s, squared, referring to the two electrons of helium in the 1 s 1s 1 s 1, s orbital. Helium has two electrons, so it can completely fill the 1 s 1s 1 s 1, s orbital with its two electrons. This can be written out in a shorthand form called an electron configuration as 1 s 1 1s^ 1 1 s 1 1, s, start superscript, 1, end superscript, where the superscripted 1 refers to the one electron in the 1 s 1s 1 s 1, s orbital. Hydrogen has just one electron, so it has a single spot in the 1 s 1s 1 s 1, s orbital occupied. The 1 s 1s 1 s 1, s orbital is the closest orbital to the nucleus, and it fills with electrons first, before any other orbital. periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number i.e., the total number of protons in the atomic nucleus. The first electron shell, 1n, corresponds to a single 1 s 1s 1 s 1, s orbital.


 0 kommentar(er)
0 kommentar(er)
